

 Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us
Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us

|

 Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us
Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us
|
| About Us | Products | Research and Development | Publications and Press | Clinical Trials | Latest News | Contact us |
 |

01/04/2014
AN ONE
MEDICINE APPROACH FOR LEISMANIASIS
Introduction
The number of emerging and reemerging infectious diseases and pandemic threats at the animal-human interface is increasing. In recent past the world has witnessed the emergence of novel diseases such as Nipah virus in Malaysia, intercontinental spread of Severe Acute Respiratory Syndrome (SARS), Highly Pathogenic Avian Influenza virus H5N1 and Influenza H1N1.
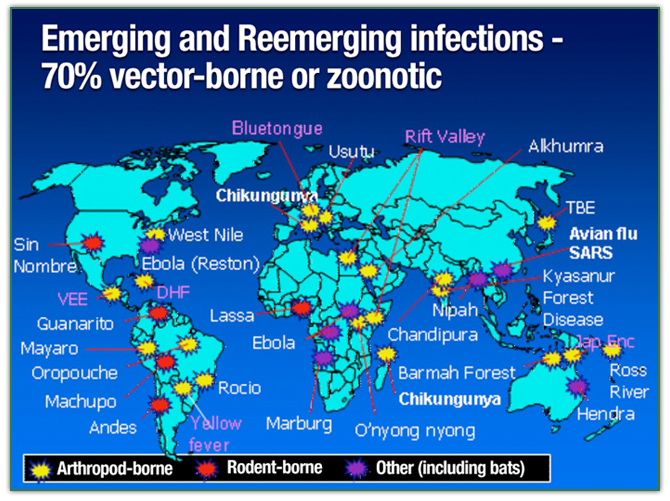
These disease events have heightened worldwide public awareness of the multidimensional linkages between wild animals, livestock production and global public health. Human population pressures and the enhanced mobility of people, climate change, food and agricultural dynamics, and the progressive encroachment of forest and game reserves, are among the more frequently cited global factors amplifying emerging infectious diseases events.
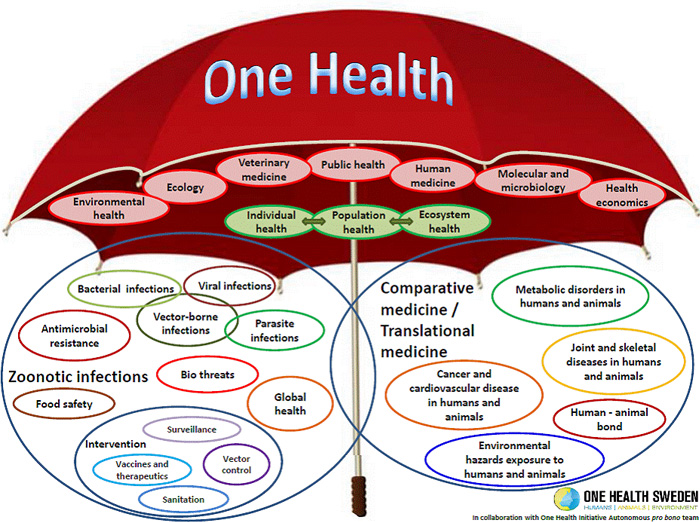
A new approach has been devised to address the multiple factors influencing the emergence of infectious diseases: The One Health approach:
This aproach can be defined as a collaborative, international, cross-sectoral, multidisciplinary mechanism to address threats and reduce risks of detrimental infectious diseases at the animal-human-ecosystem interface. In summary, the One Health concept is a worldwide strategy for expanding interdisciplinary collaborations and communications in all aspects of health care for humans, animals and the environment.
Leishmaniasis - An Overview
Leishmaniasis is a vector-borne disease caused by obligate intramacrophage protozoan parasite of the genus Leishmania. It is endemic in less developed countries and in some regions of the Mediterranean basin, including Spain, France and Italy; however, its incidence is increasing in non-endemic areas due to changing patterns of international travel and to population migration.
Leishmaniasis, exists as a spectrum of clinical disease, from cutaneous sores to disseminated disease with organ involvement. This disease, is the cause of thousands of deaths and disease in less developed countries.It is one of several diseases caused by over 20 species of Leishmania;it is transmitted mainly by sandfly bites.Cutaneous and mucosal leishmaniasis cause scarring, destruction of the mouth and nose, and severe disability.
There are 12 million cases of Leishmaniasis including cutaneous, mucocutaneous and visceral leishmaniasis currently reported. The annual incidence of Visceral leishmaniasis (VL ) is around 500,000 new cases.
Typically, patients with visceral leishmaniasis present with fever, cough, abdominal pain, diarrhoea, epistaxis, splenomegaly, hepatomegaly, cachexia, and pancytopenia. Positive serology does not guarantee clinical disease, however. Induction of visceral leishmaniasis seems to be a classic interplay of genetic background and environmental factors, as malnutrition, drug use, immunosuppressive drugs, childhood and, especially, HIV co-infection, are all associated with disease presentation.
The number of co-infections will continue to rise, notably in India and Brazil, where the urban HIV epidemic and the endemic rural visceral leishmaniasis are increasingly coming into contact. Cases of co-infection are seen as an imported disease in non-endemic areas. Co-infected patients may be difficult to diagnose, respond poorly to treatment, and relapse repeatedly.
An One Health Approach for Leishmaniasis
The dog is the main urban reservoir for human visceral leishmaniasis (VL) caused by Leishmania infantum which is listed among the most important neglected tropical diseases by the WHO. In this setting, the convergence of people, animals, and environment has created a dynamic in which the health of each group is interconnected. Thereby, an one health approach may be useful to fight this disease.
Control strategies
Control strategies are adressed to the two main epidemiological entities: anthroponotic, when human beings are the sole reservoir, and zoonotic, when dogs are the major source of infection for the vector. In either situation, efficient case management based on early diagnosis and treatment is the key to limit morbidity and prevent mortality.Effective treatment of patients is also a measure to control reservoir and transmission in anthroponotic foci, which are thought to act as a long-term reservoir of the disease. Finally, vector control should be implemented as soon as possible.
The disease spreads: Leishmaniais in US
The first US outbreak in dogs, affecting a Foxhound kennel was reported in 1999, and currently 23 US states have seropositive Foxhounds. As sandflies are the vector in many countries globally, four species of sandflies in the US are potential vectors. However, this vector source does not appear to be the main cause of transmission to humans or animals. There have been very few reports of transmission to other animal species or humans despite contact with seropositive Foxhounds. The containment of the disease within Foxhounds and a few other foreign-borne dog breeds suggests that vertical transmission is the primary form of the diseases transmission in the US.
Tough a hight number of soldiers and civilian workers have come back from Iraq and Afghanistan with leishmaniasis, and many have brought home service dogs and adopted dogs that may also be seropositive. Tough efforts are being made to address the disease in soldiers, no such effort has been made to monitor dogs coming into the US.
Cutaneous leishmaniasis is endemic within south-central Texas affecting humans, cats, and dogs. In addition, multiple vectors and rodent reservoir hosts exist within Texas, leading to consideration of vector-borne sand fly based transmission as the primary means of disease spread in this area. As researchers pointed, this facts represents a serious need for a one health approach in US to this problem, because the disease will have to pervade canine populations and begin to affect human.
Treatments
The current drugs for the therapy of leishmaniasis remain the pentavalent antimonials, which have been in clinical use for leishmaniasis for over 50 years. Other drugs includes pentamidin, amphotericin B and lipid-associated amphotericin B, paromomycin, miltefosine.
Treatment of HIV-co-infected patients present special problems; they respond slowly to treatment and relapse rates are about 60% in the first year, whichever drug is used.There have been no trials of secondary prophylaxis. The use of highly active antiretroviral therapy may control relapses.
All the above cited drugs require parenteral administration. Paromomycin (PMM) has recently been introduced for treatment of visceral leishmaniasis in India. Its acts synergistically with antimonials in vitro and in vivo. This medicine also requires parenteral administration.
Although no clinical resistance has yet been reported, recents experiments have shown resistence to PMM in vitro concerning cloned antimony-resistant L. donovani field isolates from India and Nepal.
These results may have important relevance for ensuring the long-term efficacy of PMM used alone or in drug combinations, because relapses after inadequate treatment selects resistant mutants, which are recycled in anthroponotic foci with high rates of transmission.
Miltefosine, a drug that was originally developed as an oral antineoplastic agent, is the first effective orally administered treatment for visceral leishmaniasis in humans and animals. Nevertheless, some problems persist that may limit use of miltefosine for treatament of dogs, which is the main urban reservoir. The drug is expensive, it is a potential teratogen and is toxic to male reprodutive system in dogs.
Concernig the use in humans, the treatment is expensive and requires 28-day treatment. Major limitations include low compliance, with risk of resistance, and contraindication in pregnancy and mandatory contraception for women of child-bearing age for the duration of therapy and 3 months beyond. Finally, a recent study in Asia indicated an emerging lack of efficacy in monotherapy in the region.
The long half-life of miltefosine and its narrow therapeutic index also might favour the emergence of resistant mutants. In addition, reports indicates that the therapy with miltefosine in dogs alone acts on Leishmania replication, but does not suppress the parasite in lymph nodes despite disappearance from blood of treated animals.
In view of this, combination therapy should be considered, to delay the emergence of resistance against the new drugs, including miltefosine and paromomycin. Unfortunately, the treatment of reservoirs such as dogs-- the main urban reservoir in South America and other countries--is very difficult for several reasons. For instance treatment of canine visceral leishmaniasis shows low efficacy with drugs successfully used for human visceral leishmaniais therapy, whereas the sacrifice of infected dogs is often not accepted for social reasons.
The need for new drugs and treatments
The occurrence of drug-resistant strains of Leishmania sp. in animals and human emphasizes the urgent need for developing new drugs, including those capable of boosting the bodys´ immune response.
Immunotherapy can represent a valuable approach to infectious diseases since clinical outcomes, evolution and successful treatment of leishmaniasis in dogs and humans is thought to depend, at least in part, on alterations in the host immune response to the parasite. Thereby, in animals and humans direct manipulation of the immune response, either alone or in combination with other drugs, might be a useful strategy for improving treatment regiments to prevent drug resistance.
A promissing strategy is to combine chemotherapy with immune-modulation aiming to initial elimination of parasites with chemotherapy, followed by modification of the patients immune response by an immune-enhancing or immunomodulatory agent could lead to quick recovery and control of persisting parasites.
In this setting, clearly new drugs are wellcome. The immunodulator P-MAPA is a valuable drug candidate to play this role.
Rationale for use of immunotherapy with P-MAPA in visceral leishmanisis in animals and humans under an one health aproach.
The reasoning underlying the proposal of immune-based therapy for infectious diseases using the immunomodulator P-MAPA is that an ineffective cell-mediated immune response is a characteristic feature of many diseases caused by a variety of intracellular pathogens, including visceral leishmaniasis,viral infections and tuberculosis.
In this setting, IFN-γ is well-recognized as a cytokine capable of inducing macrophages to kill intracellular Leishmania. Indeed, studies in untreated patients with VL demonstrated that the addition of IFN-γ as immunotherapy accelerated parasitological control and enhanced the clinical efficacy of conventional antimonial therapy.
Accordingly, a re-establishment of immunocompetence associated to use of P-MAPA by increasing interferon-gamma (IFN-γ) levels and decreasing IL-10 levels, has been consistently observed in animal models for infectious diseases and other pathologies,resulting in host protection and high survival rates of animals.
The compound also binding and activates cellular receptors ( toll-like receptors- TLR-2 and 4) in vitro and in vivo. These findings are of paramount importance, since is widelly known that an efficient immune response against intracellular pathogens is critically dependent on rapid detection of the invading pathogen by the innate immune system and the coordinated activation of the adaptive immune response of the hosts. Activation of toll-like receptors (TLRs) nowadays is consider the starting points for activation or re-activation of the innate immune system.
Because of this, compounds that mimic pathogen associated molecular patterns and activate immune cells via TLRs are candidate drugs being developed worldwide to treat several diseases and to be used as vaccine adjuvants. The immunomodulator P-MAPA acts as toll-like agonist in vitro and in vivo.
Experimental data from animal models for study of cancer have revealed the multiple ways by which P-MAPA can act on immune system. P-MAPA activated pattern recognition receptors (PRRs) - specifically, Toll-Like Receptors 2 and 4 (TLR-2 and TLR-4) in vitro and in vivo - and induced a cascade of immune-mediated effects responsible for the restoration of imunocompetence observed in animal models.
In addition there is evidences from previous studies that the drug candidate is a safe drug. It has not shown significant toxic effects on mice, rats, dogs, monkeys nor in pilot trials in humans. Finally, P-MAPA has great potential to be used in association with other drugs.
Taken toghether, this data pointed to P-MAPA as a valuable new weapon to act against visceral leishmaniais both in the humans and animals considering an One Healh approach to fight the disease. Indeed results from a veterinary clinical trial with the immunomodulator P-MAPA shows that the drug candidate is able to recovery partially the cellular imunity in dogs infected by Leishmania sp. The results are shown as follow:
Veterinary clinical trial in dogs infected with visceral leishmaniais
Twenty mixed breed adult dogs of both sexes, that presented antibodies reactive to Leishmania antigens, were provided by the Araçatuba Zoonosis Control Center (Centro de Controle de Zoonoses, CCZ), Araçatuba, SP, Brazil. The dogs were maintained in individual enclosed kennels, with access to water and balanced canine feed ad libitum, belonging to the Araçatuba Faculty of Veterinary Medicine (FMVA-UNESP), SP, Brazil.
The dogs were identified, weighed, received a deltamethrin-based repellent collar and, prior to the onset of the tests, were administered two doses of the dewormers pyrantel pamoate and praziquantel with an interval 15 days. They were selected based on positive serological tests for visceral leishmaniasis by ELISA and the presence of at least three clinical signs of the disease and were divided into two groups, 10 in the control group and 10 in the group treated with P-MAPA.
Skin and peripheral blood samples were collected from both groups of dogs before the onset of the experiment (day 0) and following its completion (day 45). The skin biopsies were performed with aid of the tranquilizer acepromazine (1.1 mg/kg) and local blockage with 1% xylocaine using a 4 mm "punch". The skin samples were obtained from the middle portion of the ear and stored at -80 °C until processing. Blood samples were collected by puncture of the radial vein into tubes with and without EDTA, which were used to perform the serological, hematological, biochemical, cell viability and immunophenotyping tests.
Ten dogs received 15 doses of the immunomodulator (2.0 mg/kg) intramuscularly, and 10 received saline as a placebo. Dogs in the treatment group were administered P-MAPA at a dose of 2.0 mg/kg of bodyweight, intramuscularly in the dorsal region, every three days for 45 days. The control dogs were administered saline under the same inoculation schedule.
On the inoculation days, all the dogs were observed for clinical signs compatible with canine leishmaniasis, including fever, skin lesions, lymphadenomegaly, muscular atrophy, pale mucous membranes and weight loss. Skin and peripheral blood samples were collected following administration of the immunomodulator.
The groups were followed to observe for clinical signals of remission; parasite load in the skin biopsies using real-time PCR, the cytokines IL-2, IL-10 and IFN-γ in the supernatant of peripheral blood mononuclear cells stimulated in vitro with either total promastigote antigen or phytohemagglutinin measured by capture ELISA, and changes in CD4+ and CD8+ T cell subpopulations evaluated by flow cytometry.
The use of P-MAPA in this study was approved by the Ministry of Agriculture, Livestock and Supply of Brazil under protocol no. 322, CPV/DFIP
Veterinary clinical trial : Results
Comparison between the groups showed that treatment with the immunomodulator P-MAPA promoted improvement in clinical signs and a significant reduction in parasite load in the skin. In peripheral blood mononuclear cell cultures, supernatants showed a decrease in IL-10 levels and an increase in IL-2 and IFN-γ.
Prior to the onset of treatment, all the dogs presented clinical signs of canine visceral leishmaniasis and the most common symptoms were the presence of onychogryphosis (90.0%) and lymphadenopathy (78.8%), followed by alopecia and low weight (77.3%), seborrhea and dry keratoconjunctivitis (47.0%).
Less frequently, skin ulcers, lameness, dull coat, vomiting, appetite loss, skin nodules, diarrhea, uveitis, skin rash and lethargy were observed. Regarding clinical staging, each dog received a specific score,the highest corresponding to the most severe form of the disease and high positive antibodies levels.
The distribution of the dogs was as follows: 55% were stage III and 45% stage II. Following treatment, the dogs in the treated group presented improvement in their coats, reduced flaking and itching of the skin, together with an important increase in appetite and lower clinical score (p < 0.05).
In contrast, the majority of the nontreated group presented a worsening in clinical status over time and one dog died due to kidney failure.
An increase in CD8+ T cells was observed in peripheral blood. In addition, the in vitro leishmanicidal action of P-MAPA was investigated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and no leishmanicidal activity was detected.
At the end of treatment, the dogs treated with P-MAPA showed a 100-fold reduction in the parasite load observed at the beginning of treatment. Additionally, compared to the control group, P-MAPA treatment stimulated five times more production interferon gamma (IFN-γ), which is responsible for activating immune cells and inducing resistance to the protozoan. Finally, the treatment also reduced the quantity of interleukin 10 (IL-10), which is capable of deactivating defense cells and allowing proliferation of the pathogen.
Additional studies revealed that Leishmania-infected dogs showed a decrease in TLR2 in macrophages compared with healthy dogs and in induction with P-MAPA. ROS were increased in PBMCs from Leishmania spp.-infected dogs compared with healthy dogs and P-MAPA improved ROS production. NO production was increased in culture supernatant from macrophages stimulated by P-MAPA in both healthy and Leishmania spp. infected dogs. Furthermore, treatment of macrophages from healthy dogs with immunomodulatory P-MAPA induced p38 MAPK and IKK phosphorylation, suggesting signal transduction by this pathway.
Conclusions
Considering an one health approach for treatment of visceral leishmaniais these experimental findings indicates that P-MAPA has great potential as an adjuvant immunotherapeutic drug in the treatment of visceral leishmaniasis both in humans and animals, since it assists in reestablishing partial immunocompetence of infected hosts.
References
Acta Tropica 2013: A veterinary clinical trial performed in Brazil shows that the immnomodulator P-MAPA is able to act against visceral leishmaniasis

International Immunopharmacology 2014: This study shows the effects of P-MAPA immunomodulator on Toll-like receptor 2, ROS, nitric oxide, MAPKp38 and IKK in PBMC and macrophages from dogs with visceral leishmaniasis

BMC CANCER 2016: Increased toll-like receptors and p53 levels regulate apoptosis and angiogenesis in non-muscle invasive bladder cancer: mechanism of action of P-MAPA biological response modifier



|