

 Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us
Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us

|

 Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us
Home |
Site Map |
Add to Favorites Link |
Send page to a Friend |
Contact us
|
| About Us | Products | Research and Development | Publications and Press | Clinical Trials | Latest News | Contact us |
 |

10/07/2008
• Effect of P-MAPA on murine renal carcinoma
The tumor model
Murine renal carcinoma (RENCA) has appeared spontaneously in BALB/c mice, and has been originally isolated by Dr. Sarah Stewart at the National Cancer Institute, USA. Its growth has been characterized in syngeneic animals.
The renal carcinoma (RENCA) is mildly to moderately immunogenic, and can be injected in the orthotopic site; it becomes highly vascularized as it develops, and can locally invade and form metastases in regional lymphonodes, lungs and liver (Murphy and Hrushesky, 1973).
Its progressive growth from the orthotopic site, similar to human renal cancer, makes it one of the main models for assessment of chemo and immunotherapeutic approaches to renal cancer treatment (Wiltrout, 1993; Wigginton and Wiltrout, 2002).
The renal carcionoma (RENCA) can be transplanted to a variety of anatomic sites according to the therapeutic approach to be studied.
The subcutaneous (s.c.), intradermic (i.d.), intraperitoneal (i.p.) and intrarrenal (i.r.) routes are widely employed for RENCA cell inoculation.
In general, growth rates of the resulting primary tumors are similar, with exception of the i.p. tumor, which develops faster.
Accordingly, survival time of i.p. inoculated mice (28-32 days) is slightly shorter than for the other inoculation paths (35-45 days).
In the RENCA model, different therapeutic approaches can be assessed against a progressively more advanced disease; against a disease located in different organs; as well as against a primary tumor developed in the kidney and spontaneously disseminating to other organs.
This kind of experiment demands surgical skills. Animals can be treated with the primary tumor still in the kidney.
After about 7 days of inoculation, the tumor begins to invade locally, and from day 11 on metastases appear.
In some experiments, a resection of the primary tumor (nephrectomy) is done before chemotherapy or immunotherapy is used in order to treat the disseminated residual disease.
The location of the primary tumor in the kidney makes resection relatively simple and mimetizes the clinical situation of a patient bearing a renal tumor who has the organ removed (nefrectomy) before treatment of the metastatic phase of the disease (Wiltrout, 1993).
The RENCA spontaneously invades the lungs from an i.r. implant an interesting aspect, since the lungs are one of the main metastasis targets of human renal cancer. During the progressive growth of an i.r. implant of RENCA cells hepatic metastases can also be observed.
This model is also used for the study of hepatic metastases, as they are a common occurrence during the progression of several types of human cancer, including renal cancer.
Intravenous (i.v.) injection of RENCA cells induces formation of lung metastases, whereas intrasplenic injection leads to hepatic metastases.
The advantages of these experiments for the assessment of therapeutic approaches are the relatively short duration, and reproducibility of the tumor growth.
The main disadvantage is the apparently stable clinical state of the host mouse when therapy is started generally 3-10 days after tumor injection (Wiltrout, 1993).
• Effect of P-MAPA on murine renal carcinoma (RENCA)
Experimental protocol
Animals were i.r. inoculated with RENCA cells, and i.p. treated with P-MAPA after nefrectomy (Figure 1).
Figure 1
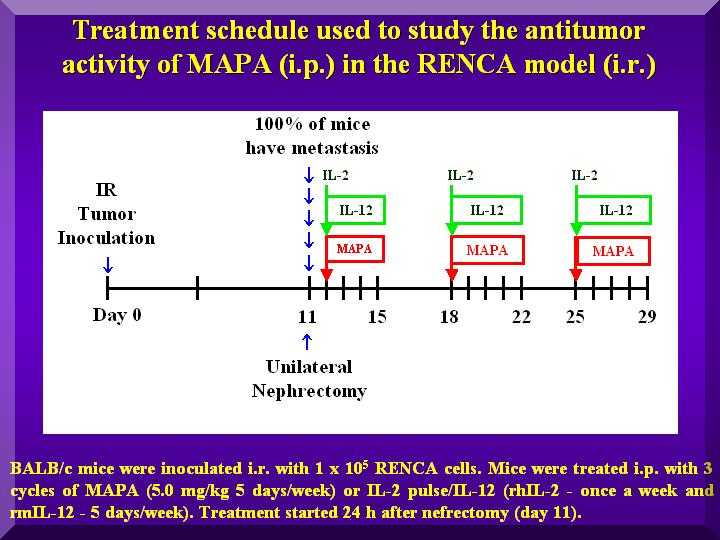
This experiment used the i.r. route for inoculation of RENCA cells in mice and the nefrectomy procedure, simulating the condition of human renal cancer.
A variety of P-MAPA dosages were used, rather than just the optimum dosage established in previous studies (Justo et al., 2000, 2003).
Animals were i.r. inoculated with 1x10e5 RENCA cells in 0.1 mL (day 0); on day 11 nefrectomy of the injected kidney was performed.
On the following day, i.p. treatment was started with P-MAPA doses in the range of 0.1-10.0 mg/kg in 0.2 mL, 5 times a week for 3 weekly cycles.
No toxicity clinical symptoms were apparent in any of the dosages.
Results
The treatment of the positive control group (IL-2/IL-12) resulted in a 40% survival rate.
Among the tested dosages, 5.0 mg/kg exhibited a statistically significant activity compared to the negative control (p = 0.0025). Results for dosages of 1.0 and 2.5 mg/kg were not statistically different from negative controls (Figure 2).
Figure 2
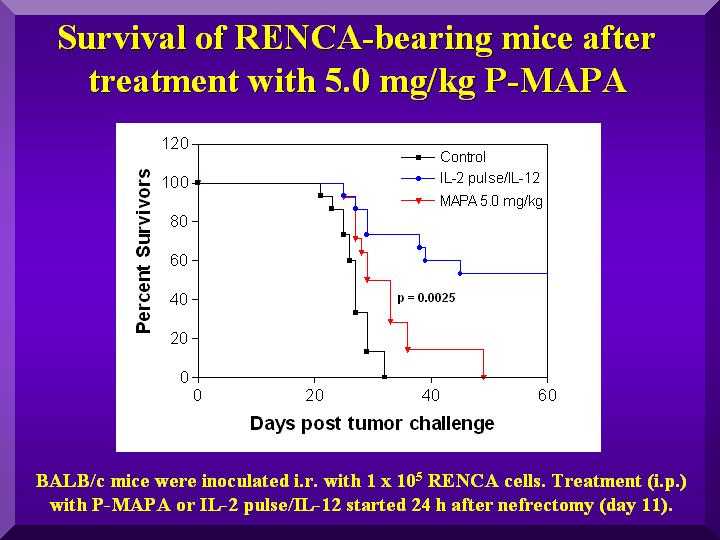
These results corroborate previous works describing 5 mg/kg as the ideal dosage for mice (Justo et al., 2000, 2003).
Due to the surgical procedure, animal weight was monitored from the day preceding nefrectomy until day 19 after it.
Whereas negative control animals did not recover their weight after surgery, animals treated with various dosages of P-MAPA and with IL- 2/IL-12 did (Figure 3).
Figure 3
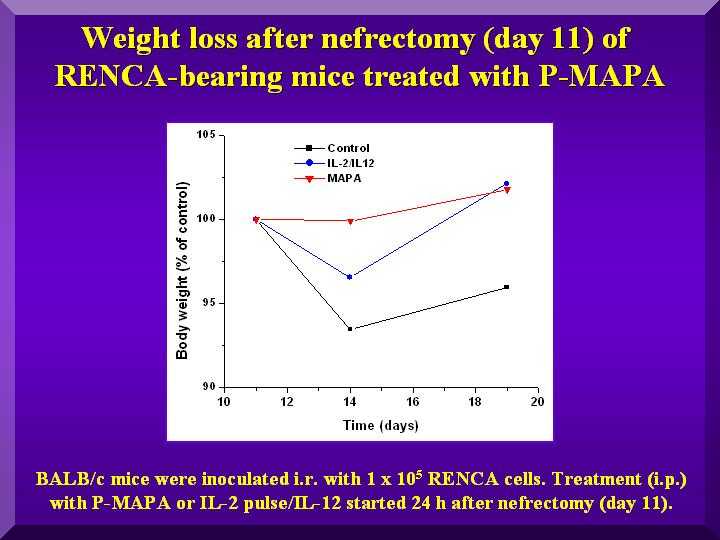
All groups displayed a higher weight loss in the 5 days following nefrectomy, as expected in a post-surgery period.
The group that showed the least weight change in the period (0.1% compared to controls) was the group treated with 5.0 mg/kg of P-MAPA, indicating a better general condition of the animals.
Number of metastases
After the death of each animal, a necropsy was done to assess metastasis distribution. Metastases were classified in a five-level scale, and results were expressed as percentages of animals with each level.
The Graph presents the results of abdominal, lung and hepatic metastases found in the negative controls and in the group treated with 5.0 mg/kg of P-MAPA (Figure 4).
Figure 4
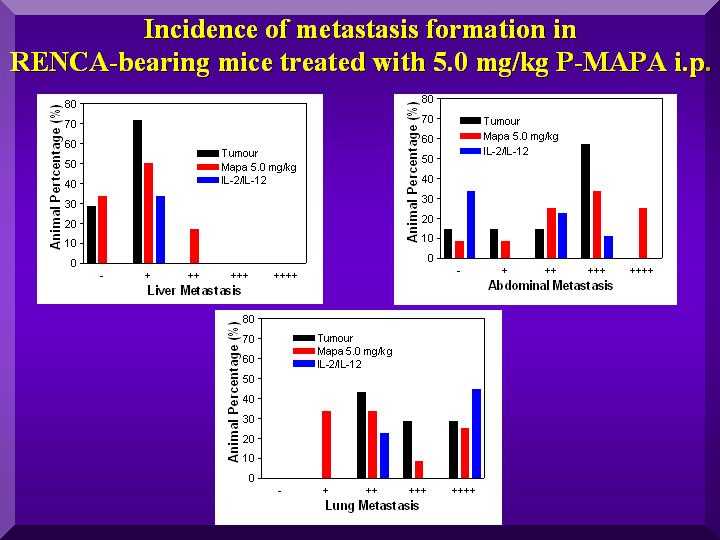
The graph show a reduction in the number of hepatic and lung metastases after administration of 5.0 mg/kg of P-MAPA to the animals.
However, no such effect was observed for abdominal metastases. In fact, data indicate an increase in the number for the treated group in comparison to negative controls.
As described in the literature, no formation of any type of metastasis is observed in non-treated animals until stage II of the disease, around 15 days after tumor inoculation.
In the period between days 22 and 24 after inoculation, classified as stage III, almost all animals display metastases in the abdominal lymphonodes.
However, lung and hepatic metastases are only observed in stage IV, after 24 days of inoculation (Salup et al., 1985; 1987).
Conclusions
• Results obtained in this experiment suggest the possibility of P-MAPA preventing formation of lung and hepatic metastases in this experimental model, and contributing to a clinical improvement of animals in stage IV, an advanced stage of the disease.
• For residual disease of renal carcinoma, treatment with P-MAPA alone or in combined therapy is promising.


|